After Ed Pigott and colleagues published their patient-level reanalysis of the STAR-D results this past summer in BMJ Open, it seemed that this scandal—which is a tale of research fraud—might finally be reported on by the mainstream media, and thus burst into the American consciousness. But eight months have now passed, and while the Psychiatric Times, much to its credit, did publish a cover story of the reanalysis, American newspapers have remained mute, even though Pigott and colleagues have contacted reporters at The New York Times and other major newspapers, urging them to set the record straight.
And now The New York Times, rather than report on the BMJ Open paper, has once again trotted out the fraudulent results as evidence of the efficacy of antidepressants.
On Thursday, April 25, The New York Times published a story titled “What You Really Need to Know About Antidepressants.” And then it informed its readers the following:
“The largest study of multiple antidepressants—nicknamed the STAR*D trial—found that half of the participants had improved after using either the first or second medication that they tried, and nearly 70 percent of people had become symptom-free by the fourth antidepressant.”
That is the bottom-line outcome that the STAR*D investigators promoted to the public in November 2006, when it published a summary of the study outcomes in the American Journal of Psychiatry. And here it is 18 years later, and The New York Times is telling of that outcome, even though it has been conclusively shown, in a paper published in a prestigious medical journal, that if the STAR*D investigators had adhered to the protocol, they would have reported a remission rate of 35%.
Although Pigott and colleagues are continuing to analyze the patient-level STAR*D data, and have already spotted evidence of a failure to report on a suicide in the study, this article in The New York Times is a sign that the scandal is on its way to disappearing from public sight, with most of the public—as a result of an extraordinary and inexplicable journalistic failure—never hearing of it.
As such, it is now a story of a twin institutional failure. The American Psychiatric Association, through its American Journal of Psychiatry, has defended the published result, and mainstream newspapers have failed to inform the public of the BMJ Open paper, letting the nearly 70% remission rate remain in the public mind of evidence of the “efficacy” of antidepressants.
The cost to society of this twin failure is extraordinary.
As a society, we organized our thinking around a “narrative of science” that, beginning in the 1980s, told of how researchers had discovered that chemical imbalances were the cause of major psychiatric disorders and that a second generation of psychiatric drugs fixed those chemical imbalances, like insulin for diabetes. This was a story of a great medical advance, and the announced results from the STAR*D trial, heralded by the NIMH as the “largest and longest study ever done to evaluate depression treatment,” fit into that story of medical progress, for it told of 70% of depressed patients becoming “symptom free” after repeated treatments with antidepressants.
However, it is not a story of advance that has survived the test of time. The low serotonin theory of depression fell apart long ago, and Ed Pigott and colleagues published their first report on the STAR*D transgressions in 2010, telling of protocol violations that had been employed to inflate the reported remission rate. Their paper in BMJ Open, if it had been reported on by the media, could have spurred a day of public reckoning: why had we been misled in this way about the results from the very NIMH trial that was expected to guide clinical care?
That possibility promised a new way forward. But now, with The New York Times publication on April 25, there is reason to think there will be no day of reckoning, and a research paper that should be retracted will continue to govern public understanding of the “efficacy” of antidepressants.
As such, this is a scandal that now tars American journalism, and not just psychiatry.
A Brief Recap of the Scandal
When the STAR*D study was launched, it was understood that that its results would guide future clinical care of depressed patients. While there had been numerous industry-funded trials of antidepressants, those trials utilized inclusion-exclusion criteria that prevented most “real-world” patients from entering the studies. Various studies had found that 60% to 90% of patients with depression couldn’t participate in industry-funded trials of antidepressants. STAR*D would fill this void.
The STAR*D investigators wrote: “Given the dearth of controlled data [in real-world patient groups], results should have substantial public health and scientific significance, since they are obtained in representative participant groups/settings, using clinical management tools that can easily be applied in daily practice.”
The STAR*D study was not designed to measure the effectiveness of antidepressants against placebo. It was designed to assess how well real-world patients fared in clinical care that utilized antidepressants as a first-line treatment, with prescribers able to change dosages and try a second, third, or fourth antidepressant if an initial treatment didn’t work.
Moreover, it had two phases. Once patients remitted after acute treatment with an antidepressant, they were whisked into a follow-up trial to assess whether their remission could be sustained with continuing use of antidepressants. The acute phase of the trial would assess whether antidepressant use could produce a moment of remission (at the end of one of the stages of treatment), while the follow-up would assess whether such treatment enabled them to stay well.
In their protocol, the STAR*D investigators told of the critical importance of this follow-up phase. “How common are relapses during continued antidepressant treatment in ‘real-world’ clinical practice?” the STAR*D investigators wrote. “How long [are remitted patients] able to stay well?”
In the first months of 2006, the STAR*D investigators published three reports in the American Journal of Psychiatry detailing remission rates following the first two stages of acute treatment. Then, in November 2006, they published a summary report of outcomes. Their paper was titled: “Acute and long-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report.”
Anyone who had read the first three articles could only be confused by this November report. The number of “evaluable patients” had changed in each of the reports; there was no report on remission rates according to a HAM-D assessment of symptoms, which in the earlier reports had been presented as a primary instrument for doing so; and although the abstract didn’t mention this, the authors confessed in their discussion that the reported 67% remission rate was a theoretical calculation, based on the notion that if those who had dropped out during the acute phase of the study had instead stayed in through all four stages of treatment they would have remitted at the same rate as those who did stay in through all four stages.
In other words, close readers of the paper would understand that the reported 67% remission rate in the abstract was something of a made-up number.
In this paper, the STAR*D investigators also reported on the one-year outcomes. However, that section of the summary paper was very short, and the investigators didn’t tell of the percentage of remitted patients who stayed well during the one-year follow-up, even though the protocol had stated that assessing the sustained stay-well rate was the primary purpose of this second phase of the study. All they stated in their section on “longer-term outcomes” was that relapse rates were higher for those who had taken several treatment steps in the acute phase of the study to remit than for those who remitted after the first treatment stage. There was a table that, in its title, told of relapse rates for patients who entered the follow-up study in remission, but it was nearly impossible to decipher, and so the bottom-line result—how many patients remitted and stayed well and in clinical care for one year—was missing from this report. Here is the table that was published:
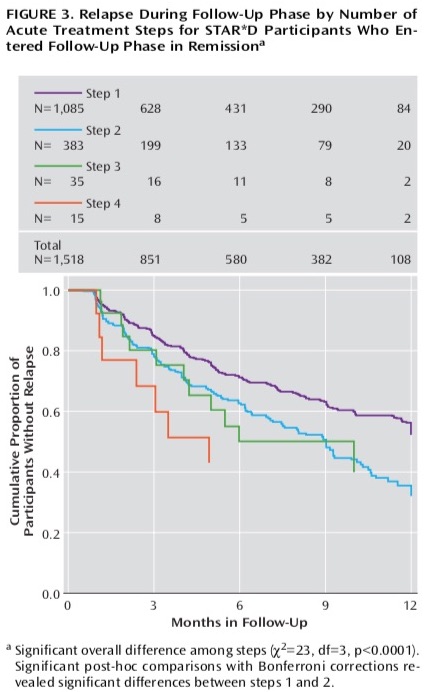
This was the very confusion that prompted psychologist Ed Pigott and colleagues to obtain the protocol through a Freedom of Information Act request and see if they could make sense of why this stream of four papers didn’t tell a coherent story. In 2010, they published an article that told of how the STAR*D investigators had violated their protocol to inflate the reported remission rate.
At that time, Pigott and colleagues reported that only 38% of the patients who met inclusion criteria had remitted after four stages of treatment. Furthermore, and most important, they made sense of the confusing graphic that the STAR*D investigators had published in their summary report that told of the outcomes in the one-year follow-up. Their finding was stunning: only 108 of the 4,041 patients (3%) who had entered the trial had remitted and stayed well and in the trial to the end of the one-year follow-up. All of the others had either never remitted, remitted and then relapsed, or dropped out at some point during the study.
This was, in fact, the most important result from the STAR*D Study. In a study of real-world depressed patients, there was no evidence that regular clinical care with antidepressants helped patients get well and stay well. That 3% figure—if it had been promoted to the public—would have prompted a rethinking of the use of these drugs.
However, few in the public were hearing of Pigott’s work. Instead, they were hearing of how the STAR*D study had proven that antidepressants were quite effective for most people.
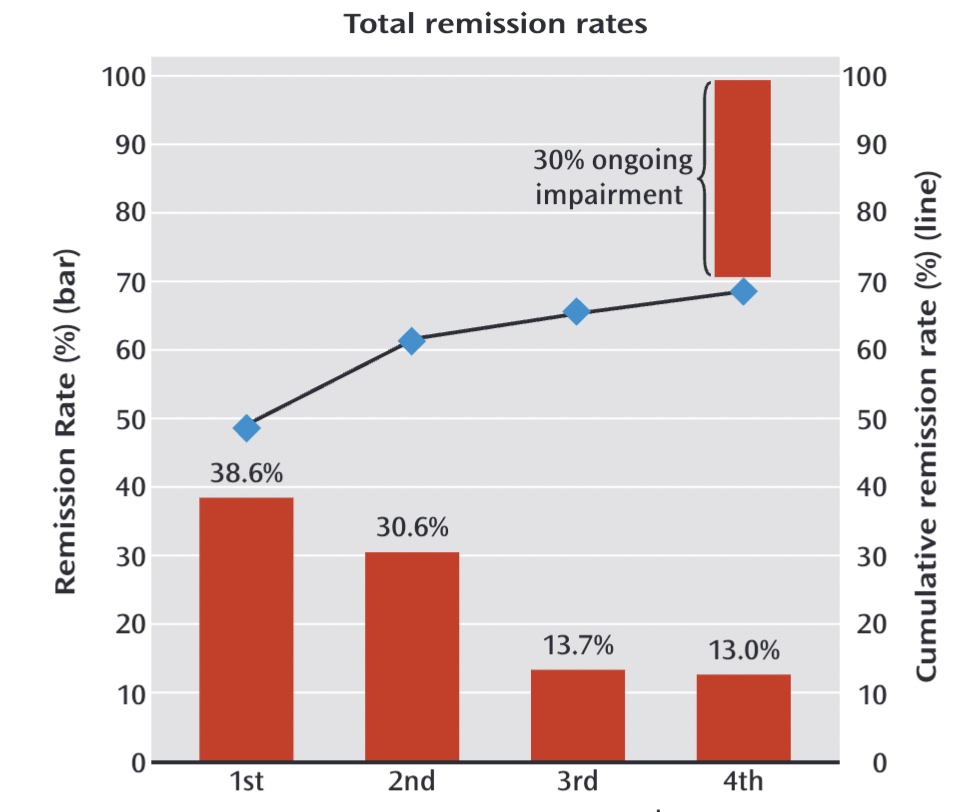
“Over the course of all four levels, almost 70 percent of those who didn’t withdraw from the study became symptom free,” the NIMH had informed the public when the November 2006 paper was published in the American Journal of Psychiatry.
The NIMH press release made no mention of the 3% sustained remission outcome, and soon that “almost 70 percent” remission rate became the result known by the public. Moreover, it was presented as the cumulative remission rate at the end of one year, which indicated that those who had remitted had remained well. Here is a graphic, published in 2013 in the American Journal of Psychiatry, that told of how there was “ongoing impairment” in only 30% of the patients.
Given that public soundbite and promotion of the 70% remission figure in the psychiatric literature, the 2010 paper by Pigott and colleagues told of a scandal. The STAR*D investigators had grossly inflated the reported remission rate and done so though various data manipulations that violated their own protocol, and equally important, they had hidden the fact that almost no patients remitted and then stayed well and in the trial to its one-year end.
Over the next few years, Pigott and colleagues published several more articles deconstructing the STAR*D results. Mad in America published two of his blogs and reported on his journal publications, and he provided Mad in America with STAR*D documents, including the protocol, that we posted on our website. Readers could see for themselves how the STAR*D investigators had violated their own protocol. Yet, no mainstream media reported on this scandal.
However, when Pigott and colleagues published their patient-level analysis of the STAR*D outcomes in July 2023, it seemed that surely this was now a story that the media could no longer ignore.
Their “reanalysis” was published in a prestigious medical journal (BMJ Open), and it detailed a scientific scandal of epic proportions. In September, MIA published its report on the scandal, and in it, we provided for the lay public a precise numerical accounting of how the STAR*D protocol violations inflated the remission rate. Here were the four principal ways they had inflated their results:
Not counting early dropouts as treatment failures
The protocol called for patients who failed to return after their baseline visit, when they were first prescribed an antidepressant, to be deemed “intolerant” to the treatment and thus counted as treatment failures. However, in their final report, the STAR*D investigators removed the 234 patients who had failed to return after their baseline visit from their tally of evaluable patients (thus decreasing the number of treatment failures).
Including ineligible patients in their count of remitted patients
The protocol required patients to have a baseline HAM-D score of 14 or greater (the scale used to measure the severity of depression symptoms.) However, in their final 2006 report, the STAR*D investigators included 931 patients who either lacked a baseline HAM-D score (324 patients) or had a baseline HAM-D score less than 14, and thus weren’t depressed enough to be eligible for the study (607 patients). By including this group of 931 patients in their final report, they added 570 to their tally of remitted patients.
Switching outcome measures
The protocol stated that the primary outcome would be an HAM-D assessment of symptoms administered in blinded fashion by a Research Outcome Assessor at the end of each of the four stages of treatment. Remission was defined as a HAM-D score of 7 or less, and anyone who scored in remission at the end of a treatment step was whisked into a year-long follow-up to see if they would stay well for this longer period of time.
In addition, the protocol stated that at each clinic visit patients would self-assess their symptoms, using a tool known as the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR). This would be done to see how well the QIDS-SR scores correlated with HAM-D scores, with the thought that in the future QIDS could become a useful tool for quickly assessing symptoms during a clinic visit. The protocol specifically stated that QIDS scores collected during clinics would not be used to report outcomes.
However, in their summary report of outcomes, the STAR*D investigators did not report remission rates based on HAM-D scores. They only reported remission based on QIDS scores. This switch of outcome measures added 195 patients to their tally of remitted patients.
Calculating a “theoretical” remission rate
The protocol called for those who dropped out of the study without having remitted to be counted as treatment failures. However, in their summary report, the STAR*D investigators theorized that if the drop-outs had stayed in the study through all four steps of the study they would have remitted at the same rate as those who did stay to the end, and they added these imagined remitters to their final tally of remitted patients. This theoretical calculation transformed 606 treatment failures into treatment successes.
In their BMJ Open paper, Pigott and colleagues reported that if the STAR*D investigators had adhered to their protocol, they would have announced that 1,089 of 3,110 eligible patients had remitted (35%.) Their four protocol violations removed 234 failures from the final tally of evaluable patients and added 1,371 to the tally of remitters, and in that manner, they produced a much different bottom line calculation: 2,460 remitters in a population of 3,671 evaluable patients (67% remission rate.)
In short, the protocol violations and imagined remissions accounted for 56% of the remissions that led to the announced cumulative remission rate of 67%.
After we published our September MIA Report, we put up a petition on change.org calling for the American Journal of Psychiatry to retract the study. More than 2,700 people signed the petition, which we sent to Ned Kalin, editor in chief of the American Journal of Psychiatry, on October 10.
We saw this as a defining moment for American psychiatry. Would the American Journal of Psychiatry do the right thing and retract the study? We also saw it as a defining moment for mainstream media in the United States: would they finally report on this extraordinary scandal in America medicine?
A Scandal Handed on a Platter to Newspapers
Pigott’s report, once it was given this public push by MIA, did trigger a brief flare-up of attention to the scandal. In December, the American Journal of Psychiatry published a response by the STAR*D investigators to Pigott’s work, and more importantly, Psychiatric Times made Pigott’s re-analysis its cover story that month. In his essay, John Miller, editor-in-chief of Psychiatric Times, wrote that since 2006, the STAR*D study had stood “out as a beacon guiding treatment decisions,” and he succinctly identified what was now at issue:
“For us in psychiatry, if the BMJ authors are correct, this is a huge setback, as all of the publications and policy decisions based on the STAR*D findings that became clinical dogma since 2006 will need to be reviewed, revisited, and possibly retracted.”
At that point, Ed Pigott and his colleagues contacted The New York Times and other mainstream publications. The essay by Psychiatric Times had laid out what was at stake for the public, and it was nothing less than guidelines for treating depression and our societal understanding of the efficacy of antidepressants. BMJ had validated Pigott’s reanalysis, and now a psychiatric journal had validated its importance.
When the Psychiatric Times cover story was published, I thought that the media’s silence on this subject would surely now be broken. This was a story being handed to reporters on a ready-made platter. You had documents that told of the protocol violations. You had a patient-level reanalysis published in a prestigious medical journal that detailed how the STAR*D investigators had employed protocol violations to grossly inflate the reported remission rate. You had a psychiatric journal telling of the importance of this study, and how, if the reanalysis was correct, the profession had been led astray by the NIMH-funded investigators and had adopted prescribing protocols that were not warranted.
This was a big story! A story, in fact, that would stun readers in countries around the world, given the importance of the STAR*D study to the global psychiatric community. And when Psychiatric Times subsequently published a letter from two psychiatrists titled “STAR*D: It’s Time to Atone and Retract” it seemed that surely newspapers would jump on the story. The two authors of the letter, Nicolas Badre and Jason Compton, told of the extraordinary influence of the STAR*D study, describing it as probably the “most important study” in the “recent history of psychiatry.”
They then provided these examples:
“The results of STAR*D are familiar to all psychiatrists as commonly tested on boards and residency training exams. According to its abstract, the main conclusion of STAR*D was that ‘the overall cumulative remission rate was 67%.’”
“The impact of STAR*D was outstanding; it is highly cited in our textbooks. The latest edition of Tasman’s textbook of psychiatry makes 54 references to the STAR*D trial and includes a full chart of the study.”
“Similarly, the latest edition of the widely read Maudsley’s prescribing guide makes 9 references to STAR*D . . . More than 100 peer-reviewed articles have been written about STAR*D, and the original paper was cited 207 times on PubMed.org in 2023—the most of any year.”
“Newspapers have regularly promoted its findings and continue to do so. As recently as last year, The New York Times was citing STAR*D as ‘the largest study of antidepressants to date,’ and touting its results that more ‘than 60 percent of those patients actually had a very good response.’ ”
“Some may think finding that antidepressants are effective in 67% of patients is trivial; however, the efficacy of antidepressants was not as widely accepted prior to STAR*D. The saturation of psychiatric textbook with STAR*D, more than ever before, solidified that teaching.”
For a journalist, that letter tied a red ribbon around the entire story. There were documents to base the story on, there was a paper published in a prestigious journal that detailed the protocol violations and the gross inflation of the reported remission rate, and there were psychiatrists to interview who could tell of how the inflated STAR*D results had misled their entire profession and the public.
And yet, so far, the mainstream media has remained mute. And now, on April 25, The New York Times, rather than turn its attention to the scandal, repeated the very falsehood that has poisoned the psychiatric literature and its textbooks since 2006. It did so in an article that promised to tell readers the “facts” about antidepressants.
That story told of a remarkable journalistic failure. I submitted the following comment to the article:
“The New York Times needs to inform its readers that the STAR*D investigators violated their own protocol, in four ways, to dramatically inflate the remission rate of nearly 70% that they reported in their 2006 paper. A re-analysis of patient level data by Ed Pigott and colleagues, which was published in BMJ Open last July, found that if the STAR*D investigators had adhered to their study protocol, the true remission rate at the end of four stages was 35%, not 70%. Please do a story on this re-analysis; the public needs to be informed of the true results from this NIMH-funded trial, and not be misled, yet again, by this report of a 70% remission rate in this NIMH study. The story of the STAR*D trial is a story of research fraud, and the paper published in the American Journal of Psychiatry that told of that fraudulent result needs to be retracted.”
The New York Times moderator did not allow that comment to be published.
An Intent to Deceive
In their letter published in the American Journal of Psychiatry, John Rush and four of his STAR*D investigators defended their work, stating that in a study like STAR*D, it was important to provide results for all of the patients in the study, and chided Pigott for removing 931 patients from their analysis. They wrote:
“The analytic approach taken by Pigott et al. has significant methodological flaws . . . in total, 941 patients included in our original analyses were eliminated from Pigott et al.’s reanalyses based on their post-hoc criteria. The rationale for removing these participants from the longitudinal analysis appears to reflect a studious misunderstanding of the aims Rush et. al. paper, with the resulting large difference in remission rates most likely the result of exclusion by Pigott et al. of hundreds of patients with low symptoms scores at the time of study exit.”
And:
“Effectiveness trials by design aim to be more inclusive and more representative of the real world than efficacy trials. By removing the data of over 900 study participants from their reanalyses, Pigott et al. failed to recognize the purpose of inclusiveness. It appears that the authors created rules to define post hoc which subjects to include, which eliminated many subjects who experienced large improvements during one or another of the study’s levels. By doing so, the sample is biased to underestimate the actual remission rate.”
This letter, by itself, tells of an intent to deceive. It is a stunningly dishonest letter, and the editors of the American Journal of Psychiatry, having published the STAR*D reports in 2006, were surely aware that it was so. There is no acknowledgement in Rush’s letter that the 931 patients excluded by Pigott et al. either weren’t depressed enough to qualify for the study or lacked a baseline HAM-D score. They hid this essential fact, and instead claimed that Pigott and colleagues had created “post-hoc criteria” to wrongly remove them from their re-analysis.
While this is an extraordinarily brazen lie, it is easy to understand their motivation for coming up with this “defense” of their work. If they had told of how these 931 patients were excluded because they didn’t have baseline scores that made them eligible for inclusion, American Journal of Psychiatry readers would immediately understand that the STAR*D investigators, by including them in their final tally of “evaluable patients,” were guilty of research misconduct.
The STAR*D investigators were of course duty bound to present results based on their protocol for assessing outcomes. However, if they had also wanted to report remission rates for the 931 patients who didn’t meet eligibility criteria for the study, they could have done so in a transparent manner and that would have been fine. Their summary paper should have told of the inclusion criteria, and in addition to reporting remission rates based on their protocol, the STAR*D investigators could have stated “and here are the remission rates for the 931 patients who hadn’t met eligibility criteria for the study.”
But there is no such transparency in their 2006 report. Instead, there is evidence, at every step, of an intent to deceive.
First, here is how they presented their results in the abstract:
“Results: The QIDS-SR16 remission rates were 36.8%, 30.6% 13.7% and 13.0% for the first, second, third, and fourth acute treatment steps, respectively. The overall cumulative remission rate was 67%.”
That paragraph informs readers that the QIDS was the primary outcome measure, and that 67% of the patients entered into the study remitted. The abstract is omitting these facts: that 931 of the patients in in this summary report didn’t meet eligibility criteria, that the HAM-D was designated as the primary outcome measure, and that this 67% is a “theoretical” number that relied on converting study dropouts into imagined remitters.
Second, in their “methods” section, they did not mention that patients needed to have a baseline HAM-D score of 14 or higher to be eligible for the trial. Instead, they wrote this:
“Participants met DSM-IV criteria for nonpsychotic major depressive disorder at study entry as determined by clinical diagnosis and confirmed with a DSM-IV checklist by the clinical research coordinator.”
Now, in their report on remission rates after the first stage of treatment they noted that patients needed to have a HAM-D score of 14 or higher to be eligible in the trial, and thus they concluded that 931 patients who lacked a baseline HAM-D score or had a HAM-D score less than 14 were not “evaluable patients.” But in this summary report, they snuck them back into the roster of evaluable patients, and they did so by implying that the DSM-IV checklist for major depressive disorder was the instrument used to assess eligibility.
Third, they did not disclose that the protocol had declared that the HAM-D would be used to assess whether a patient had “remitted.” Instead, the STAR*D investigators wrote:
“We used the Quick Inventory of Depressive Symptomatology—Self-Report (QIDS-SR16) as the primary measure to define outcomes for acute and follow-up phases.”
That declaration made it seem that they had planned to use this tool for reporting outcomes all along. They did not note that the protocol called for the use of HAM-D to assess remission rates, and they did not confess that the protocol explicitly stated that the QIDS-SR, which was administered at clinic visits, was not to be used to assess research results. Furthermore, they did not report HAM-D remission rates in this paper.
Fourth, as noted above, in their presentation of the one-year outcomes, they did not tell of how many patients stayed well and in the trial to its end. Instead, they wrote: “Relapse rates were higher for those who entered follow-up after more treatment steps.”
Those are the footprints of deception that can be found in their summary paper of outcomes, and it was a deception that accomplished the following:
- It turned a 35% remission rate after four stages of acute treatment into a “bottom-line” finding that with multiple trials of antidepressants eventually 67% of depressed patients became symptom free.
- It hid the extraordinarily poor results from the one-year follow-up trial, which told of how very few patients had remitted, stayed well and in the trial to its one-year end.
When the STAR*D investigators defended their 2006 by report stating that they wanted to be “inclusive” of all patients, there was a way they easily could have done that. They simply would have needed to first report outcomes based on the protocol for patients who were eligible for the trial, and then they could have added a section on remission rates for those who didn’t meet eligibility criteria. Similarly, they could have reported remission rates based on the QIDS-SR, but they would have first needed to report remission rates based on the HAM-D. They would also have needed to confess that the protocol stated that the QIDS-SR would not be used to report research results.
Such transparency would tell of an effort to honestly report results. Instead, the “footprints of deception” visible in the published article tell of an intent to deceive, and thus provide evidence of scientific misconduct.
Will the American Media Step Up?
At this point, American psychiatry, as an institution, seems content to let this story die down. The American Journal of Psychiatry is not going to retract the paper, and that really isn’t a surprise: American psychiatry has shown, again and again, that it is devoted to telling the public a story that maintains societal belief in the merits of its treatment, and there is a long record of negative results, such as the results from this STAR*D trial, being kept from the public.
But now it is The New York Times and the mainstream media that are facing a defining moment in their coverage of American medicine: will the media do its duty and inform the public of this scandal? Or will it, for reasons that are difficult to understand, remain mute? At this point, it appears that the media will take the latter course, and by doing so, fail the American public in a profound way.
***
MIA Reports are made possible by donations from MIA readers like you. To donate, visit: https://www.madinamerica.com/

The NYT article is very poor for many reasons. I made this comment yesterday to the article and as of this morning the comment was still there with 28 likes, a relatively small number so most people will never see it.
Unfortunately this article contains misinformation. The Star*D trial has been criticized for violating its study protocols. Ed Pigott and colleagues published a patient-level reanalysis, in BMJ Open, that found that if the STAR*D investigators had adhered to their protocol, the true remission rate at the end of four stages was 35%. But it’s even worse. Pigott found that the actual number of people who stayed remitted and continued to the end of the trial was dismal—108 of the 4,041 in the trial, or about 2.7%. That’s because a huge percentage of the STAR*D participants dropped out of the trial, with over a thousand participants dropping out during their first trial of antidepressant. Many of these participants counted as having “remitted,” despite the fact that it’s usually people who do poorly or have adverse effects who drop out of studies.
In addition, side effects from antidepressants are not rare. For example, up to 73% of antidepressant users experience sexual dysfunction. This article does not do justice to the numerous controversies surrounding antidepressant use.
Report comment
This is outrageous.
The falsified STAR*D results have sent millions of people down an ineffective path. Moreover, they hinder recovery and remission that could have happened otherwise. They might have created increase in morbidity and mortality rather than decreasing it, due to significant side effects. The subsequent trend of widespread, long-term prescriptions of antidepressants has probably fuelled the chronicity of depression and devastating, debilitating withdrawal symptoms. In simple words, this publication helped to kill people and destroy lives.
We need to keep pushing the truth forward. We need to keep telling and retelling it over and over again.
Thank you, Robert. Thank you very much.
Report comment
Once again, the mainstream media fails us. I read that NYT article and my mouth dropped open at the accumulation of pharma talking points….instead of the inclusion of scientific findings. And then to see they refused to publish your comment? Money talks and pharma money seems to drown out all reason. Many of us know the truth about the psych drugs, but because of stories like the ones in the NYT, we are often viewed as the cranks……the curse of Cassandra.
Report comment
The mainstream media no longer operates in the public’s best interest. It now operates at the behest of Big Pharma, because that’s one of its biggest paymasters.
All you need to do is take note of how much ad space goes to Big Pharma!
Report comment
The mainstream media operates at the behest not only of Big Pharma but also other major corporate entities. In short, what we have is corporate fascism, oligarchy, or “inverted totalitarianism,” as journalist Chris Hedges defines this corrupt system. The misnamed “mental health” industry as a whole serves to legitimize and perpetuate its warped neoliberal values.
Report comment
Hi Ann—I’m
Reading your book in Vision therapy. My vision was impaired by psychiatric treatment. Trying to heal my iatrogenic injury reading a harmed patients autobiography seems somehow poetically appropriate to me. Thank you for sharing your story. Here’s mine https://www.madinamerica.com/2023/01/can-barely-breathe/
Report comment
Hi Blu, Thanks for getting in touch. I hope you enjoy the book, and I’ll be sure and read your story. Courage as you move forward! Ann
Report comment
I wonder if the pharmaceutical companies threatened to withhold advertising money if they would have reported accurately….
Report comment
BINGO!!!
Only a fool would think Big Pharma isn’t pulling the purse strings with the NYTimes—along with every other mainstream media outlet.
Report comment
I was also wondering about the role of advertising in what is being reported.
Report comment
I copied the last two paragraphs of Robert’s article and posted on the comments section of the NYT’s article and suggested a full read of it on MIA. No surprise it didn’t get published as that would be biting the hand that feeds it.
Duplicate comment.
Report comment
The problem isn’t just that antidepressants (as well as other psychiatric drugs) aren’t nearly as effective as Big Pharma would have us all believe.
The bigger problem (imo) has to do with how frequently, casually, and needlessly these drugs are prescribed.
Most doctors pay little to no attention to the risks involved in taking these drugs, risks that include not just transient side effects, but also the possibility of permanent iatrogenic harm/illness and/or protracted withdrawal.
As things stand now, informed consent is usually inadequate at best, if it occurs at all.
Report comment
Wow that was unreal ..the angles and stories they come up with in defending themselves and this starD “dodgy” representation of the facts- are the best in the business of obscuration and manipulation like magicians, that was as good and as convincing a clear and true account of what they did..excellent reporting..write .. clearly dodgy- caught out- on “so many” counts and levels, and still there- lying about the count.. counting people not in it..gone..imagining cause they liked the drugs so much- they didnt need any more…or something- not very clear about it-yep!- slithering snakes in the grass – watch out- i hear the bites, neuro- toxic..
Report comment
Well, decades ago the estimable Thomas Szasz wrote a book aptly titled “Psychiatry: The Science of Lies.” I would just add that psychiatry shouldn’t be dignified by even calling it a science; rather, it’s a racket, a gigantic scam, a modern-day cargo cult financed by Big Pharma and enabled by corrupt shills in the media, academia, and so-called regulatory bodies.
Report comment
I copied the last two paragraphs of Robert’s article and posted on the comments section of the NYT’s article and suggested a full read of it on MIA. No surprise it didn’t get published as that would be biting the hand that feeds it.
Report comment
Hi Gerard & Everyone else,
I tried posting my comment twice now in response to this article and it has not been posted. I really don’t understand why. I made reference to Dr. Grace Jackson’s “What Drs Don’t Tell You About Psychiatric Drugs” which is available on the internet, I also suggested people take a look at Dr. Witt-Doerring’s YouTube channel and I praised Dr. David Healey’s RxISK website.
Guess what? The NY Times did not publish it. I have no idea why since many others shared their comments which were truthful and critical of these medications.
So I submitted a comment which was meant as an inquiry and request for them to print my comment.
I’ll paste it below. If anyone has an idea of why it was rejected please share your feedback. Thank you.
Anonymous
Canada
Pending Approval
Good Evening,
I wrote you a comment which is very important I feel to alert readers of the NY Times about the little mentioned but very real sideffect of akathisia from SSRI’s. Please do me a favor and publish it. It is truthful, it happened to me and your readers should be made aware of this life altering and potentially life ending devastating sideffect- that is mentioned in the list of side-effects from drugs like Effexor and Zoloft- under the heading “restlessness”. Well akathisia is restlessness on steroids…every waking hour of the day. And the days turn into years. Please publish my comment as l’ve seen several others here since | wrote mine.
Thank You- mine is just as relevant.
Report comment
‘biting the hand that feeds it’ sums of the New Yawk times motivation here. I can’t say I am surprised, since Noam Chomsky’s book Manufacturing Consent: The Political Economy of the Mass Media has been in print since 1988.
Report comment
Yet my comment, which was very critical, was published (see what I wrote above). I also responded to a primary care doctor who commented that he was aware of the facts in the article and discussed them with his patients. I told him that was unfortunate because the article had so much misinformation in it. After a brief delay, that, surprisingly, was published.
I don’t know why some comments get in and others don’t. A while back, I had a comment in the NYT published and then removed a couple of days later despite numerous likes. I think use of words like fraud, or arguing that facts are being kept from the public will almost guarantee rejection.
Report comment
My comments were not approved either. I’ve been monitoring the comments for three days.. Although there are a surprising amount of comments criticizing the piece, nearly all of the ‘readers picks’ are for comments in support of tge article. ‘Readers Picks ‘ is usually a measure of which comments get approved first.
Report comment
Mr. Whitaker,
Don’t know if you’re aware….Propublica is beginning to investigate Mental Health in the U.S.
I was interviewed over a month ago. While I am a subscriber to their newsletter, have enormous respect for their journalism, and witnessed the impressive effects of their efforts nationally and locally (West Palm Beach), I was very alarmed by what seemed to be a focus on currently reported Gen Z struggles finding support/care.
They were missing the big picture….the (lack of) SAFETY AND EFFICACY of that support/care-QUALITY CONTROL of the industry writ large.
I named you & your books (and Star*D) as essential touchstones…and “Medicating Normal” (minus the bloviating by A. Frances) among many other resources.
I don’t think I was an effective vessel for this critical message. I think I missed an opportunity. On the other hand, could have been massive hubris on my part to think I knew ‘best’…for them. It was upsetting.
I felt like like ‘Deep Throat’ in the dark parking garage telling Redford (Bob Woodward/WAPO)…”You’re missing the overall, the big picture…follow the $”…and lies.
I probably sounded crazy…(humor).
If you would like additional deets (reporters name/contact, etc), happy to share.
You have my email.
Report comment
Thank you for the investigative reporting.
Report comment
The fact that the mainstream media won’t expose the fraudulent STAR*D study to the American public speaks volumes. Since SSRIs hit the market, the mainstream media has been complicit in the iatrogenic epidemic created largely by the pharmaceutical companies and psychiatry.
When we look back at this time in history, we will remember SSRIs as one of the biggest scams in medical history. The journalist who hid the truth about these drugs from the public will remain on the wrong side of history.
Report comment
I also subscribe to Psychiatric Times, keeping in touch with the industry. (‘Know your enemy, know yourself’ Sun Tzu)
The recent article(s) suggesting that the time for Star*D criticism to be acknowledged, accepted, and addressed in prescribing practice was astonishing…considering their editorial philosophy of always supporting psychiatry’s fragile legitimacy.
One would think it would spark a plan of ACTION from prescribers, sparing clients the widely acknowledged damages (manufacturer warnings!) from these addictive drugs….and future liability charges.
Report comment
And because psychiatrists, NPs, GPs & all other western allopathic doctors still do, shows us what?
1. Maybe that they are in business to make money first and foremost
2. Maybe the real job isn’t to heal people but to create repeat customers
Report comment
Bob,
As you well know, the NYT has always been promoting the medical model of mental health and has proactively ignored any contrary valid science. So this is not surprising. Mainstream media, unfortunately, has lost its credibility in these and many other important areas of daily life. Many of its ex-reporters are on substack and other alternative sites. We are at a critical juncture in a society where the public has no clear way to gather valuable news, political or any other. Our schools have failed to produce good critical thinkers, and the media has fallen in lockstep with the powers that be in promoting misinformation to keep everyone in line unquestioningly.
Report comment
I watched the White House Correspondence Dinner last night. It wasn’t a celebration of frivolity but watching a wake without alcohol. The room was quiet. Nobody laughed at the really funny jokes. The heaviness of the crowd permeated through the television. The majority of what President Biden said was a plea for media to start, well, telling the truth. The sensationalism and biases that has caused an exodus of consumers to social media.
With every positive there is a huge negative. I would never had known about this barbaric abuse by psychiatry and its unholy alliance with BigPharma without YouTube. Without Google I would have never know about MIA. I also found a lot of other platforms willing to tell the truth because they didn’t have rely on advertising by BigPharma.
With the transgender debate coming to a fever pitch. The closing of Tavistock in the UK and research reporting by Lisa Littman. There is a paper that was written by David Schellenberger exposing the “expert” organization on transgender affirming treatment. The lack of efacy research, long term studies, and the lack of transparency to all the harms caused by hormone blockers, cross hormone therapy, and the surgery is coming grossly more apparent.
Again, this wasn’t covered well by left bias media. Those outlets spewed the lies the there was no harm in puberty blockers. The lie that stopping puberty blockers wouldn’t cause permanent damage. All are false.
While the UK has banned gender affirmed medical intervention until the age of consent. More Republican states are also passing legislation to stop the same treatments. More of these stories are not widely covered by US conventional media. Left wing is scaring parents into submission with the threats their children would commit suicide. What a horrific tactic that is. More right wing platforms are covering it more.
When I look at the explosion of transgenderism it starts to creep into psychiatry about the time psychiatry started to called out by Psychiatric Survivors and a greater flooding on social media of the fraud of the chemical imbalance theory and a greater recognition of the harms of psychiatric medication. There was more uncovering of the complicity of the FDA and basically our government’s complete lack of accountability.
Also as the transgender debate started to heat up there was a new flooding of an ideology run amok. We are all traumatized. Our parents were awful, society is oppressive, trauma dumping at places like fast food car pick up windows. People feel the need to tell the poor employee about how horrible our lives have been. While we can always be victims of operation or abuse it keeps us in a state of an inability to move forward in our lives. It causes inaction of finding societal cures to actually fix things. People run to their therapists and cry for all the injustice in their lives.
What is the response to unrealized trauma? Psychedelics is the new fancy pill to elucidate all that trauma within you. There are Kedamine Clinics and doctor supervised LSD trips. Now there is the upcoming discussions on low dose daily use of psychedelics. There is a pill for everything that is ailing you.
My point is that there is always a new psychiatric disaster that shouldn’t be challenged, debated or have an outside 3rd party entity doing real studies on these constantly evolving mental health societal crises.
Fortunately, our friends across the pond and Finland are starting to need for psychosocial discussions and a greater need for it. This not only addresses the issues causing people mental pain and anxiety and finding ways to fix things.
It has been well established that NAMI receives funding from BigPharma. Many larger, and louder, trans activists organizations also receive funding from BigPharma. Those activists calling for more use of psychedelics are also funded by BigPharma.
I don’t begrudge anybody’s right to take psychiatric medications. I’ve stayed on mine under the knowledge of the harms they continue to cause. I have no intention of going through withdrawal ever again. This is a question of choice with my informed consent.
I don’t begrudge any adult wishing to seek trans sexual medical care. As an adult making a decision based on informed consent it is your decision. I have apprehension on minors making that decision of a lack of informed consent. A young child doesn’t comprehend the long term side effects of this care. An 11 year old doesn’t know that having sex in the future can be or that medications can cause irreparably harmed. Surgery is forever. It has also been overwhelming acknowledged in study after study that most young people are gay. Some are heterosexual. Some are bisexual. At least these children were given the time they needed to find their sexuality.
I am a child from a traumatic childhood. I suffered serious narcissistic/psychopathic abuse. I live with devastation to my body by psychiatric medication. It was the fraud of the chemical imbalance theory that locked me in that marriage for decades. There are huge percussions of racism, rights and abuses to immigrants, the operation on women under the draconian abortion laws passed, there is such a backlash on the LGBTQ community by the abuse of a society that has spent far too long on the rumination’s of psychiatry’s narcissistic need to remain relevant after century after century and decade after decade of abuse.
The curtain will be pulled back and the wizard will be seen as the frightened, fraudulent, opportunistic and con artists that that profession has become.
As to psychedelics, that’s so embarrassingly obvious it requires no comment by me.
That what leads me back to social media. This isn’t information reported by commercial media. These reports come about by a deep dive into social media. There is still the issue of algorithms. As users of social media I tend to screw up the algorithms. I think YouTube had a meltdown when I subscribed to the Megan Kelly Show. I’ve watched Matt Walsh on occasion. I subscribe to Medicating Normal or other platforms willing to tell the truth. I frequently watch more International News, such as France24 and Deutsche Welle. We all have the ability to like, subscribe, ring the bell but also share.
That doesn’t mean that still putting pressure on mainstream media needs to stop. It would be unrealistic for this to be covered now. Palestine and Israel, Ukraine, Iran and our own election must take precedent. If our mainstream media does a lot better at covering these things we may still have a democracy. Then we can do more to stop this.
Report comment
I wholeheartedly agree, support, respect and believe my fellow transgender human travelers! Adult & child alike!! I trust them when they tell me who they are.
But I also wholeheartedly DIStrust big pharma and the FDA! I do not agree, support nor respect them. And I DO NOT believe them when they tell me who they are.
I do not think hormone blockers are safe. I do not believe a word out of any of their greedy evil corrupted mouths!!!
Blockers and every other drug and pharmaceuticals should be kept out of children’s mouths!
The FDA should be dismantled immediately and completely. We should build a regulatory system that is FOR the people, BY the people and OF the people!! And by “people” I mean ALL people. Not just rich white men! But they would definitely be included too (unfortunately)
Report comment
My comment was not published either. I unsubscribed from the NYT today.
Report comment
Okay, so the drugs are not effective. Are there consequences of stopping taking them? Withdrawal symptoms?
Then what would be the consequences of telling people the truth? Where would you look to see the effects of people stopping taking these drugs?
If there was a massive increase in homicides/suicides, would that mean that the drugs would now have efficacy? That is, people don’t die if they take them?
It would give the snake oil salespersons time to get the f*&^ outta Dodge (to quote the ‘Public Enemy’). And that’s what’s going to be important to the likes of the N Y Times.
Kick the can down the road a bit more.
Report comment
Boans
Are you suggesting ” states quo” .
Report comment
“Status quo?”.
I’m suggesting ‘they’ will do whatever is necessary to ensure that accountability is ineffective, and that includes involuntarily euthanizing people (as a last resort of course, most times the slander of ‘mental patient’ will deal with them, and the gaslighting till they suicide. Seen this done first hand so …… i’m used to the “they wouldn’t do that” mantra).
I think people would be surprised at the ‘advocates’ who are actively engaging in the concealment of the truth, and all the while touting themselves as being the defenders of the truth they are concealing. And being paid handsomely for the inside information about any problems which may arise (ie throw the client under the bus type ‘legal representatives’)
Report comment
The consequences of telling consumers the truth that a product is ineffective is that those consumers will stop buying the product. But if your newspaper tells consumers that the product their own sponsor makes is ineffective, that sponsor will cease paying you to advertise their product and then your newspaper will go out of business (even if the sponsor’s business doesn’t)
Report comment
“a product is ineffective is that those consumers will stop buying the product.”
That’s all well and good if it were a food stuff or something similar. These drugs have the tendency to cause dependency problems (maybe even addictions?). It may not be a choice for the heroin addict to stop buying the product. Is the same true of these ‘mental health medications’?
Report comment
“It is better to have questions that can’t be answered then answers that can’t be questioned” Dr. Julie Ponesse
Report comment
It’s little wonder that the NY Times is known as the American Pravda.
Report comment
This reminds me a bit of the Post Office / Horizon scandal that was recently loudly portrayed on PBS.
The problems started in 1999 with the rollout of a brand new computerized accounting system for the entire British Post Office network, which includes about 11,500 local offices. The system, designed by Fujitsu (a trillion-dollar international corporation with 124,000 employees) was buggy from the beginning. But the corporate refusal to admit that anything was wrong led to the false imprisonment of many postmasters, their financial ruin, and loss by suicide of at least 4. 3,500 Post Office franchisees were victims of this corporate crime. A high court only recently ruled that this was indeed a crime perpetrated on British postmasters, and an inquiry is still ongoing. I doubt the inquiry will ever get to the bottom of why company managers behaved so savagely against their own people. The official “owner” of the company, by the way, is the UK government.
I can only conclude that most large corporate entities eventually turn criminal. They are run top-down, they do not need to make their inner workings public, and they are capable of making a few people a ton of money. The way they are organized and the way they work are almost an invitation to indulge in criminal behavior. And all it takes is a few nut jobs holding key positions to cause worlds of hurt for everyone else. I wish we could learn from these events.
Report comment
This all makes me very sad and weary. I worked for years as a “behavioral scientist” (I hate the term) in Family Medicine Residency programs, where I directed the physician/teachers toward the large body of evidence showing that antidepressants don’t work, that antipsychotics cause more long-term problems than they solve, and that their history-taking should include much more curiosity about peoples’ psychosocial and economic context than is the custom. For the most part I was treated as a nice person who is well-intentioned, but too idealistic for the “real world” where moving product through the system, putting a simplistic label on a complex situation, and prescribing a pill is the order of the day. I take heart from the way you persist. I like to believe the numbers of those of us who believe in a better way are growing. You are certainly doing your part.
Report comment
Thank you for trying Gene.
Report comment
Thank you Gene for providing such a succinct encapsulation depicting the downstream impact resulting from institutional failure, e.g., mal-educated and mal-trained mental health professionals doing the best they can within a multi-corrupted system. Some hard and painful reckoning coming in the not too distant future.
Report comment
Great research Mr Whitaker and the reception your reporting and Mr Piggott’s research got shows that Western civilisation is in its death throes.
When a so-called trustworthy independent newspaper is covering up truths that were eventually acknowledged by the profession that pushed these drugs, you know it’s all over.
Morality, truth and justice do not exist any more or exist in tiny little pockets like this underfunded website or on the occasional podcaster on Youtube – although even those are shilling for their book or their podcast.
Scores of people have had their lives destroyed/damaged by these drugs and there won’t be any recompense. It doesn’t take a journalist – a stupid layperson can see the blatant falsehood of Star D whatever and yet NYT decided to double down on a lie. Remember that good old seventies film – ‘Three Days of the Condor’ when Redford puts the file to the NYT in the post box and tells the CIA man ‘they’ll publish it’ and he replies ‘Will they?’ or something – well we’re long past that now.
NYT are presumably being partly funded by some class of corporation that has pharma involvement (people aren’t buying newspapers much anymore). That’s probably the answer. I’m agnostic but it’s kind of biblical as in Lot and Sodom – ‘Can you find me one good man?’ Apparently not in the NYT – I can’t say I’m surprised. Roll on China’s takeover of democracy’s corpse.
Report comment
A broader issue: Considering how many people take these drugs, and the financial cost to insurers and individuals, why is a single $35 million study regarded as exceptionally “large-scale” and defining for the field?
Report comment
Robert Whitaker’s resolve to keep chipping away at powerful institutional forces is both awe inspiring and, for me at least, despairing. For the paper of record has a well established inglorious history of censorship by commission and omission from which to protect the vulnerabilities of the powerful, and a (virtual) impunity to do so at whatever constitutive expense necessary to transfer those vulnerabilities onto “vulnerable populations”, e.g., people without political capital. This is effectively the role of our institutions now, and the stenographers at NYT accomplish this role with an exceptional Orwellian aplomb. Well… maybe this new “engulfed” wrinkle will lead to some unforeseen crazy political butterfly effect that at least flushes out a future NYT response addressing the STAR*D scandal, one worthy of the 4th estate? This seems to me a realistic expectation now, however otherwise doggedly pursued until accomplished.
Report comment